DMD Hub News
News and updates with the latest clinical trials, medical reports and interesting developments within the DMD community.
Latest
- DMD gene therapy patient referral pathway recommendations published
-
New recommendations have been published to help ensure fair and timely access to gene therapy clinical trials and treatments for Duchenne muscular dystrophy patients.
- 5 September 2023
- Read More
Additional Updates
DMD Hub News
- DMD gene therapy patient referral pathway recommendations published
-
New recommendations have been published to help ensure fair and timely access to gene therapy clinical trials and treatments for Duchenne muscular dystrophy patients.
- 5 September 2023
- Read More
- DMD Hub Clinical Trial Recruitment Project continues after successful first year
-
The 31st March 2023 marked the one year anniversary since the launch of The DMD Hub Central Recruitment Database Pilot Project (CRD).
- 31 March 2023
- Read More
- First Boy in UK Dosed on Sarepta's Gene Therapy Trial at John Walton Muscular Dystrophy Research Centre
-
A boy has been dosed on the Sarepta EMBARK gene therapy clinical trial for Duchenne muscular dystrophy (DMD) at the John Walton Muscular Dystrophy Research Centre, a world-leading neuromuscular research centre run by Newcastle University and Newcastle Hospitals.
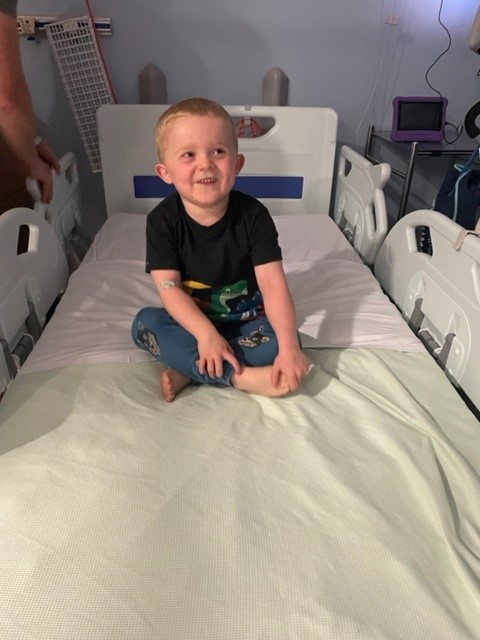
- 25 October 2022
- Read More
- Launch of the DMD Hub Central Recruitment Pilot Project
-
The John Walton Muscular Dystrophy Research Centre (Newcastle University) and Duchenne UK are delighted to announce that the DMD Hub Central Recruitment Pilot Project is now active and accepting registrations.
Register online at: www.dmdhubrecruits.org
- 1 April 2022
- Read More
- DMD Hub Impact Report 2021
-
Read about the impact of the DMD Hub in 2021 in our latest impact report.
- 16 March 2022
- Read More
- The DMD Hub Central Recruitment Pilot Project Webinar - Thursday, 31st March 2022, 7-8pm
-
On Thursday 31st March 2022, 7-8pm, Dr Michela Guglieri (Consultant Neurologist at Newcastle University and Newcastle Hospitals NHS Foundation Trust) and Dr Anne-Marie Childs (Consultant Paediatric Neurologist, Leeds Teaching Hospitals NHS Trust) will be hosting a webinar (for patients/families) to discuss the launch of the DMD Hub Central Recruitment Pilot Project.
- 14 March 2022
- Read More
- DMD Hub team expands to provide more support to patients, sites and industry
-
In the last few months, the DMD Hub has recruited two new staff members to work with Emma Heslop, the DMD Hub Manager, and to look at new areas where the DMD Hub can develop support.
- 11 November 2021
- Read More
- New clinical trial posts funded at the DMD HubÂ
-
The DMD Hub is one of Duchenne UK’s flagship projects and has successfully expanded capacity for Duchenne muscular dystrophy (DMD) clinical trials in the UK. By funding key posts at hospital sites across the UK, many more boys are now able to take part in trials for potentially life-changing treatments.
We were delighted to welcome the following new staff to the DMD Hub network. Each team member will play an important role in running effective trials for potential DMD treatments:
- 13 August 2021
- Read More
- Virtual reality trial to help children with DMD engage in physiotherapy at DMD Hub site
-
A team of researchers and clinicians from Sheffield Hallam University, Sheffield Children’s NHS Foundation Trust and Leeds Teaching NHS Trust are developing an immersive virtual reality (VR) platform to help children living with Duchenne muscular dystrophy (DMD) engage in physiotherapy exercises.
- 6 August 2021
- Read More
- Key milestone: first UK patient enrolled in DMD gene therapy trial at DMD Hub site
-
Pfizer has announced that the first UK-based DMD patient has been enrolled in a gene therapy trial at Newcastle Hospitals NHS Foundation, a DMD Hub site.
- 12 May 2021
- Read More