DMD Hub First Year Round-up
The DMD Hub was created by Duchenne UK and leading neuromuscular clinicians based at the John Walton Muscular Dystrophy Research Centre in Newcastle and Great Ormond Street Hospital in London. The DMD-Hub is addressing the lack of capacity in the UK for clinical trials in Duchenne Muscular Dystrophy. Our ambition is to ensure that every child diagnosed with DMD in the UK is given the opportunity to take part in research.
- 1 Jan 2018

During the first year of operation the DMD Hub has successfully achieved, even surpassed, the immediate priorities identified. In particular we have:
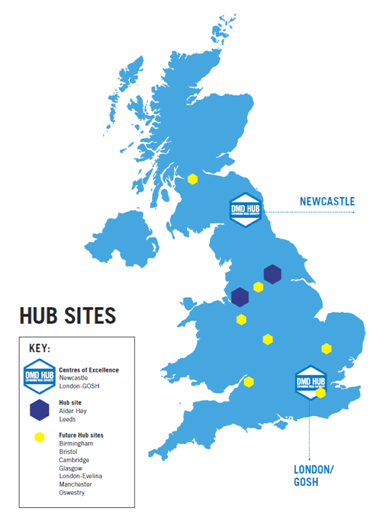
- Visited 9 key sites in the UK in order to establish a network of clinical trial sites, providing core support and advice on how to develop the infrastructure to undertake DMD trials.
- Set up Alder Hey and Leeds as the first Hub sites capable of running academic and industry led studies.
- Committed to funding posts at our next Hub site in Glasgow, further increasing the academic excellence and geographic reach of the DMD Hub.
- Engaged with additional sites (Bristol, Birmingham, Oswestry, London-Evelina, Cambridge and Manchester) to discuss how the Hub can facilitate them taking on upcoming industry and academic led trials.
- Worked with industry to promote Hub site capabilities, specifically carrying out site feasibility enquires for 3 companies interested in initiating or expanding trial capacity in the UK during 2018.
- Promoted collaborative working with key stakeholders in the neuromuscular field to develop the Hub toolbox providing resources to support trials at different stages of the clinical trial pathway.
- Utilised existing resources to enhance the work already achieved, collaborating with TREAT-NMD, North Star Clinical Research Network and the Action Duchenne UK DMD Patient Registry.
- Organised a face to face meeting with over 70 stakeholders from the UK DMD field on 18th September in Alder Hey, to update on the progress of the Hub. During the meeting we shared information and engaged in honest discussion about how the Hub can further develop to increase trial capacity. The outcomes will help develop a 5 year plan ensuring that the Hub aligns with the government’s life sciences industrial strategy.
- Worked to implement the National Institute of Health Research (NIHR) goals in rare disease using experiences of ongoing DMD trials. Several meetings with local and national representatives have taken place and progress has been made on linking investigators at sites with NIHR resources.
- Planned to establish a DMD Hub website for sharing resources and information. The website will be launched in Q1 2018, it will be a key resource for the community and will contain a repository of training material for sites and act as a one-stop shop for industry / sponsors interested in conducting trials in the UK.
- Set the groundworks for establishing the Hub as a training resource which will facilitate training for sites including physios, clinical trial coordinators and clinical research associates
- Committed an additional £400k to fund clinical Hub posts at UK sites to deliver on increasing clinical trial capacity for the DMD trials in the UK.
The DMD-Hub Steering committee have agreed a priority list for 2018 to open up further opportunities for additional sites. The priorities include setting-up an additional 2-3 Hub sites, launching the DMD-Hub website, activating training programmes and developing the Hub toolbox.
Enquiries related to the DMD-Hub should be directed towards Emma Heslop (DMD-Hub Manager) Emma.Heslop@ncl.ac.uk