First Boy in UK Dosed on Sarepta's Gene Therapy Trial at John Walton Muscular Dystrophy Research Centre
A boy has been dosed on the Sarepta EMBARK gene therapy clinical trial for Duchenne muscular dystrophy (DMD) at the John Walton Muscular Dystrophy Research Centre, a world-leading neuromuscular research centre run by Newcastle University and Newcastle Hospitals.
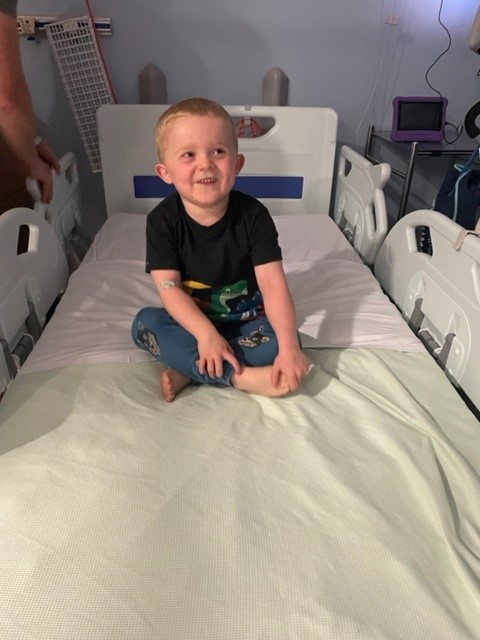
- 25 Oct 2022
Four-year-old Charlie from Aberdeen was able to take part in the study in Newcastle thanks to the work of Newcastle Hospitals, Newcastle University and DMD charity, Duchenne UK.
DMD is a severe genetic disease, mostly diagnosed in boys, which causes muscle wasting disease, leading to gradual loss of mobility and sadly limits life expectancy to around early 20s.
The trial, called EMBARK, is studying the safety and efficacy of gene therapy, a technique that works by adding healthy new copies of the gene that is broken and causes DMD.
The John Walton Muscular Dystrophy Research Centre is coordaining the DMD Hub network of sites, set up by Duchenne UK, to support the delivery of clinical trials. It is one of only two sites in the country which has centre of excellence status and has a world-renowned reputation.
Newcastle Hospitals' paediatric research team played a vital part in the trial, dosing Charlie at the National Institute for Health and Care Research Clinical Research Facility at the RVI. The facility is run jointly by the trust and university and specialises in early phase trials, usually in rare disease and conditions.
Charlie, whose nearest trial centre is 150 miles away in Glasgow, was able to take part in the trial by using a new recruitment database, co-ordinated by Newcastle University and funded by Duchenne UK as part of the DMD Hub.
Charlie’s mum, Jennifer, was contacted about the EMBARK study through the database:
“We felt that if Charlie could take part in research, we could help move science forward and benefit other little boys and their families in future. However, as he receives his clinical care here in Aberdeen, the nearest trial site in Glasgow is still 150 miles away. The DMD Hub’s central recruitment database was possibly the only way that Charlie could have a chance at participating.
"Knowing that Charlie has played just a small part in this, whatever the outcome, thanks to the opportunity the DMD Hub has given him – I have no words to describe our pride in our brave little boy!”
Professor Volker Straub, Director of the John Walton Muscular Dystrophy Research Centre, Professor of Neuromuscular Genetics at the Institute of Translational and Clinical Research at Newcastle University, and honorary Clinical Geneticist Consultant at Newcastle Hospitals, said:
“DMD is a devasting condition that sadly shortens the lives of patients and significantly impacts quality of life.
“I am incredibly proud to be leading this study, and of the teams at Newcastle University and Newcastle Hospitals who have worked together to dose the first patient on the trial.
“We are grateful to Duchenne UK for their support and look forward to working together to discover new treatments for patients with DMD.”